Tracing cerebral cortex evolution
Molecular atlases of turtle and lizard brains shed light on the evolution of the human brain
Our cerebral cortex, a sheet of neurons, connections and circuits, comprises “ancient” regions such as the hippocampus and “new” areas such as the six-layered “neocortex”, found only in mammals and most prominently in humans. But when in evolution did the components of cerebral cortex arise and how did they evolve? Scientists at the Max Planck Institute for Brain Research in Frankfurt am Main studied gene expression in the neurons of the cortex of turtles and lizards, and found unexpected similarities and differences with the mammalian cortex. These results are a milestone towards reconstructing the evolution of the vertebrate brain.

We are, in many ways, our cerebral cortex. Its circuits serve to shape our perception of the world, store our memories and plan our behavior. A cerebral cortex, with its typical layered organization, is found only among mammals, including humans, and non-avian reptiles such as lizards and turtles. Mammals, reptiles and birds originate from a common ancestor that lived some 320 million years ago. Neuroscientists believe that this ancestor had a small cortex with three layers, because a similar structure is found today in the hippocampus of mammals and in all cortices of modern reptiles: these three-layered cortices likely correspond to their common ancestral cortex.
By comparing the cortex of today’s reptiles to the old and new cortices of today’s mammals (such as hippocampus and neocortex, respectively), we can search for similarities, potential ancestral traits, and differences – resulting from their independent evolutions – and thus reconstruct the main features of cortical evolution. Comparisons were, until now, based on developmental and anatomical features. This new study, based on the molecular characterization of individual reptilian neurons, provides unprecedented data to help reconstruct cortex evolution.
For decades, the anatomical differences between reptilian and mammalian brains have fueled many disputes about cortical evolution. People argued on whether this part of the reptilian brain corresponds to that part of the mammalian brain, or whether the many layers found in mammalian neocortex actually exist also in reptiles, but in a form that is not detectable with traditional methods. Gilles Laurent and his group at the Max Planck Institute for Brain Research took a different approach and focused on the molecular characterization of the myriad neuronal types that make up cortical circuits.
Transcriptome sequenced
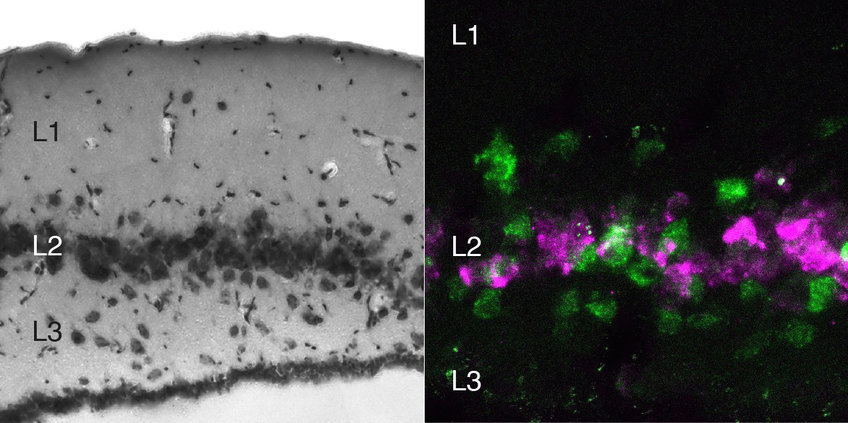
Neuronal “types” differ, among others, by their morphology, neurotransmitters, connections and functional properties. These features all result from the expression of different sets of genes; hence individual neurons can be classified (or typed) by measuring the messenger RNA molecules they contain (their “transcriptome”). Maria Antonietta Tosches, the first author of this study, and her colleagues sequenced the transcriptomes of turtle and lizard cells after capturing them, one by one, in microscopic water droplets using specialized microfluidics platforms.
Using these gene expression profiles, the scientists could categorize thousands of neurons. From each type they could identify diagnostic marker genes, and use them to assess the position of the cell types in the brain. Imagine a picture of the cortex, uniform until then, suddenly transformed into a collage of colored zones, with each zone containing one or several characteristic cell types.
The authors could now compare reptilian molecular maps to those of mammalian brains directly, find one-to-one correspondences and even draw hypotheses about the brain of their common ancestor of 320 million years ago (now extinct).
Forced to fold
“Our results tremendously clarify our understanding of the reptilian brain and thus, of brain evolution”, Tosches says. These new molecular maps show, for example, that reptiles have neuron types that correspond to those found in the mammalian hippocampus, a structure involved in spatial orientation and in the formation of memories. In reptiles, the hippocampus is found towards the center of the brain, but unlike its folded-up mammalian counterpart, looks like a single sheet. “It is as if, in early mammals, the ancestral hippocampus had been pushed by an increasingly dominant neocortex and forced to fold onto itself, to acquire its signature mammalian architecture”, Laurent adds.
The non-hippocampal reptilian cortex, by contrast, revealed the intricate history of mammalian neocortex. Inhibitory neurons, for example, express similar sets of genes in reptiles and mammals, indicating a common ancestry. Excitatory neurons, however, differ substantially across these two groups. “The mammalian six-layered neocortex is a fascinating mosaic of ancient and new neuronal types”, says Tosches. The scientists can now point to the true novelty of the mammalian neocortex, that is, the emergence of new types of excitatory neurons after profound changes of gene expression programs.
This study opens up many new questions. Do ancient neuronal types have the same functions in reptilian and mammalian cortical circuits? And can these molecular similarities and differences inform us on the evolution of brain function and animal behavior? “There is a lot more to explore from these new molecular maps” says Laurent: “this is only the beginning”.
AV/HR