Protein stock for the beginning of a new life
Cytoplasmic lattices in the egg cell supply the early embryo as protein storage sites
When mammals have offspring, they invest a lot. Unlike fish or frogs, the embryo cannot develop on its own. It has to implant in the uterus, where it is supplied with everything it needs to survive. Until this happens, the egg cell nourishes the early embryo. Among other things, it provides essential proteins. Researchers led by Melina Schuh at the Max Planck Institute for Multidisciplinary Sciences, together with colleagues in Göttingen, have now elucidated how egg cells store proteins. Their experiments also provide important insights into how errors in protein storage can lead to infertility. Structures of the egg cell that have puzzled scientists for over 60 years play a crucial role in this.

For many couples, parenthood is a long time coming. For some, the desire to have children even remains unfulfilled. The causes are varied and can be found in both men and women. In women, fertility decreases with age and infertility becomes more likely. However, genetic mutations can also contribute to this.
Analyses of the genetic material of infertile women worldwide have shown that one of the most common genetic causes of female infertility is mutations in certain genes. These contain the blueprints for the protein PADI6 and the protein complex Subcortial Maternal Complex, or SCMC for short. However, it was previously unclear what role these proteins in particular play in infertility.
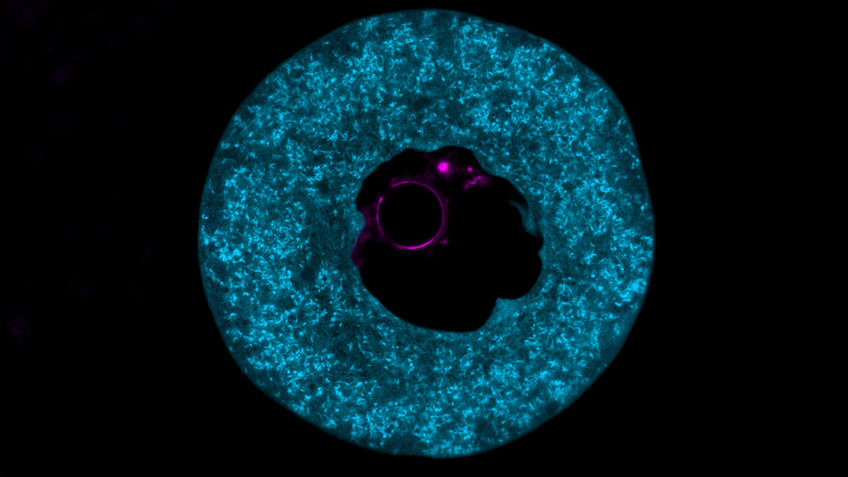
Protein storage site for the early embryo
Scientists led by Melina Schuh have now used imaging techniques to visualize that PADI6 and SCMC are the main components of structures that fill the interior of the egg cell. “The research world has been puzzling for decades about the function and composition of this structure, which we refer to as cytoplasmic lattices,” Max Planck Director Schuh explains.
When the scientists removed the PADI6 and SCMC proteins from mouse egg cells, the cytoplasmic lattice was lost – with fatal consequences. “Mouse egg cells that lacked the cytoplasmic lattices also lacked the proteins required by the early embryo. The development of the embryo came to a halt shortly after fertilization,” the cell biologist says. “We therefore suspected that the cytoplasmic lattices could serve as protein storage sites.”
Storing proteins in the egg cell is by no means trivial. This is because egg cells are created in the ovaries of a female mammal from birth and remain functional there for months or even years. Egg cells have to keep their proteins in stock for a correspondingly long time without being degraded or becoming active at the wrong time.
In the next step, the researchers investigated which proteins are contained in the cytoplasmic lattices. In cooperation with groups led by Henning Urlaub and Juliane Liepe from the Max Planck Institute, they used mass spectrometry and bioinformatics to determine the exact protein inventory of the lattices.
As the results showed, the cytoplasmic lattices bind to many proteins that are crucial for embryonic development. “Our results are strong indications that our assumption is correct: The cytoplasmic lattices are the protein storage sites of the egg cell and supply the early embryo with vital proteins,” Schuh points out.
PADI6 and SCMC proteins store proteins

As they further discovered, the PADI6 and SCMC proteins take on the task of collecting and storing proteins for the early development of the embryo. “This explains why embryos stop developing shortly after fertilization if these proteins are missing or cannot fulfill their function,” says Ida Jentoft, first author of the study. “We were then interested in whether a defective protein storage site can be replaced if, for example, PADI6 and SCMC are missing due to gene mutations.”
In experiments, the team succeeded in artificially reintroducing the missing lattice proteins into growing mouse egg cells. With this approach, it may also be possible to rebuild the cytoplasmic lattice in defective human egg cells. According to Jentoft, this could be a promising new approach to treating infertility caused by mutations in the PADI6 and SCMC genes.
Frozen egg cells under the microscope
When asked why it has taken so many decades to decipher the function of the enigmatic lattice structures in the egg cell, Schuh and Jentoft have a short answer: the size of the egg cell and the lack of methods. Egg cells are the giants among the various cell types in mammals. What predestines them for storing proteins makes it difficult to look inside them.
“The major methodological challenge was to make the egg cells accessible for the imaging methods we used – high-resolution light microscopy and cryo-electron tomography (cryo-ET). The latter makes it possible to examine molecular structures of the egg cell in 3D under almost natural conditions. This has not been possible until now,” the Max Planck Director explains. The team‘s breakthrough in this procedure was helped by a tried and tested trick from reproductive medicine.
For cryo-ET, cells must first be shock-frozen. The researchers took advantage of the fact that clinics routinely freeze human egg cells for artificial insemination to store them. To protect the egg cells during freezing, the clinics use cryoprotectants. “We had the idea of using the same cryoprotectants to achieve the required rapid freezing of the egg cells,” Rubén Fernández-Busnadiego from the University Medical Center Göttingen reports. “With this technique, we can examine the cytoplasmic lattices in the egg cell and begin to decipher their structure in detail,” Schuh adds. The scientists hope that the new techniques will lead to important advances in egg cell research as well as new therapeutic approaches in the future.