Skull bone marrow expands throughout life
Lifelong vascular growth drives increase of blood cell production
The ability of the bone marrow to produce healthy blood cells declines significantly with age, leading to age-related inflammation and disease. A team of researchers at the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has shown that the skull bone marrow is a exception to bone marrow aging and actually increases blood production throughout life. Specialized blood vessels within the skull bone marrow also continue to grow and drive this expansion, making this a unique case of lifelong vascular growth in the aging body.

The bone marrow microenvironment governs the self-renewal and fate of hematopoietic stem cells, which make all the blood cells in our body. This sophisticated and finely tuned network of hematopoietic stem cell maintenance is disrupted during aging, leading to disproportionate production of immune cells and an overall decline in their function. Blood vessels, a critical component of this niche, decrease in number and lose functional integrity in most organs during aging. Excessive fat accumulation, bone loss, severe inflammation, and a strong bias for myeloid cells over lymphocytes are also major hallmarks of the bone marrow aging.
Most bones in our body contain bone marrow, but long bones, such as arms and legs, and flat bones, such as the skull, are formed through different developmental and ossification processes. The mouse skull, due to its thin and nearly-transparent physical properties, has long been used as an intravital imaging platform to follow hematopoietic stem cell activity in the bone marrow of living mice, assuming that all bone marrow microenvironments in different bones are comparable.
Unique environment in the skull
Scientists at the Max Planck Institute challenged this assumption and asked: Do all bone marrow compartments age in the same way? They have now discovered that the bone marrow of the skull has very unique properties that allow it to continuously expand during adulthood and remain surprisingly resilient to aging hallmarks throughout life.
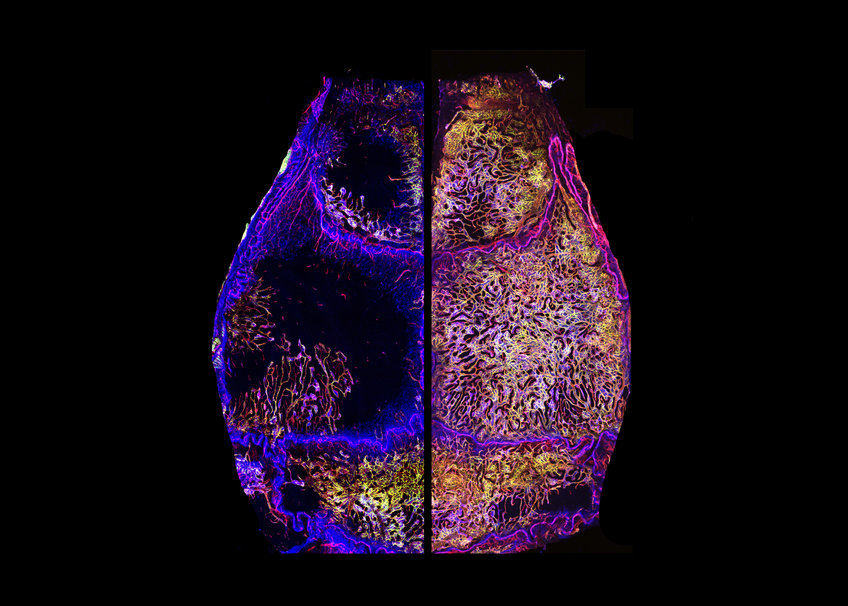
This striking observation was made when a team of Max Planck researchers led by Ralf H. Adams used a special imaging technique to visualize the entire vascular network and all the bone marrow cells in the entire calvarium, which is the roof of the skull. Using this in vivo immunofluorescence method, the scientists were able to compare the overall changes in the calvarial bone marrow during aging.
Calvarium filled with bone marrow and blood vessels
Bong-Ihn Koh, first author of the study and postdoctoral fellow in Ralf H. Adams’ laboratory, encountered a completely unexpected transformation when comparing skulls from fully-grown young adult versus 95-week-old geriatric mice: “There is very little bone marrow in the young adult calvarium and I didn’t expect to see any major changes in the total amount of bone marrow during aging. But when I looked at the skulls of geriatric mice for the first time, I was amazed to see that the calvarium was now completely filled with bone marrow and full of blood vessels.” This continuous growth of the skull bone marrow was also observed in computed tomography (CT) scans of young adults compared to old human subjects.
Using various pharmacological treatments to modulate blood vessels, the scientists discovered that continuous vascular growth in the bone marrow of the skull drives its substantial expansion throughout life. “Most vascular beds in various organs of our body would decrease in number and function during aging. The massive expansion of the skull bone marrow during aging was definitely surprising, but the substantial increase in blood vessels within the skull bone marrow was even more surprising. We think this is a very unique case of lifelong vascular growth in our body,” says Koh.
Not only did the skull bone marrow and vasculature grow substantially with age, but the hematopoietic stem cell microenvironment remained resilient to key hallmarks of aging and stayed surprisingly healthy throughout life. “All of the major hallmarks of aging observed in the femur of aging mice, such as excessive fat accumulation, inflammation and immune cell type bias, were nearly absent in the skull bone marrow of the same mice. In fact, various functional experiments ultimately demonstrated that the aging skull bone marrow microenvironment keeps hematopoietic stem cells in a better shape than in long bones,” says Koh.
Different pathways
The current study also showed several differences in the molecular pathways of hematopoietic stem cells and endothelial cells, which make up the blood vessels of the bone marrow, between the skull and femoral bone marrow of geriatric mice. “While we now know that the bone marrow of the skull continues to grow and remain surprisingly healthy during aging, we still need to figure out how this nurturing and resilient microenvironment is created and maintained,” says Koh. “This is really just the tip of the iceberg. Understanding how certain components of the niche are uniquely regulated would allow us to apply this knowledge to make other bone marrow compartments resilient to aging as well.”
This groundbreaking study is not only a significant contribution to the field of aging research, but also has the great potential to fundamentally change how we think about specialized functions between similar tissues, such as the distinct immunological function of the skull bone marrow in general physiology and various diseases.