A giant interneuron for sparse coding
A single interneuron controls activity adaptively in 50,000 neurons, enabling consistently sparse codes for odours
The brain is a coding machine: it translates physical inputs from the world into visual, olfactory, auditory, tactile perceptions via the mysterious language of its nerve cells and the networks which they form. Neural codes could in principle take many forms, but in regions forming bottlenecks for information flow (e.g., the optic nerve) or in areas important for memory, sparse codes are highly desirable. Scientists at the Max Planck Institute for Brain Research in Frankfurt have now discovered a single neuron in the brain of locusts that enables the adaptive regulation of sparseness in olfactory codes. This single giant interneuron tracks in real time the activity of several tens of thousands of neurons in an olfactory centre and feeds inhibition back onto all of them, so as to maintain their collective output within an appropriately sparse regime. In this way, representation sparseness remains steady as input intensity or complexity varies.

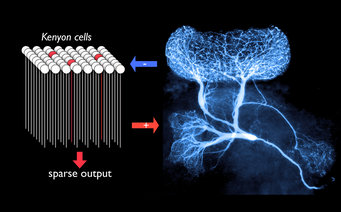
Signals from the world (electromagnetic waves, pressure, chemicals etc) are converted to electrical activity in sensory neurons and processed by neuronal networks in the brain. Insects sense smells via their antennae. Odours are detected by sensory neurons there, and olfactory data are then sent to and processed by the antennal lobes and a region of the brain known as the mushroom bodies. Neurons in the antennal lobes tend to be “promiscuous”: odours are thus represented by specific combinations of neuronal activity. Neurons in the mushroom bodies—they are called Kenyon cells—, however, respond with great specificity and thus extremely rarely. In addition, they generally respond with fewer than three electrical impulses when stimulated with the right odour. This “sparse coding” strategy has the advantage that it simplifies the task of storing odour representations in memory.
Surprisingly, each Kenyon cell is connected on average to half of all possible presynaptic neurons in the antennal lobes. So how do the Kenyon cells manage to respond only extremely rarely, and with a sparseness that varies little over large ranges of stimulation conditions? Gilles Laurent of the Max Planck Institute for Brain Research and his group found that a single giant interneuron plays a key role. Along with colleagues in his lab (formerly at Caltech) and Great Britain, he has discovered that this neuron, with its extensive arbour, is activated by the entire Kenyon cell population and in turn inhibits them all back. “The giant interneuron and the Kenyon cells form a simple negative feed-back loop: the more strongly it is activated by the Kenyon cell population, the more strongly it curtails their activity in return”, explains Laurent. The interneuron itself does not generate any action potentials, but inhibits Kenyon cells via nonspiking and graded release of the neurotransmitter GABA (gamma aminobutyric acid). This smooth, graded property enables this giant interneuron to do a kind of real-time, population averaging, thus carrying out an operation that might otherwise require the involvement of hundreds or thousands of individual spiking neurons.
The effectiveness of the giant interneuron is such that it can actually turn off the Kenyon cell population completely. But the research team also discovered that the giant interneuron is, in turn, controlled by another inhibitory neuron. “This allows the network activity to be potentiated or attenuated, and the sensitivity of this feedback loop to be adjusted”, says Gilles Laurent. This is an important feature for brain regions such as the mushroom bodies, which are responsible not only for olfactory processing, but also for learning and memory. Mushroom bodies are where smells can be associated with other sensory modalities, enabling the formation of complex representations.
The scientists’ findings show how massive negative feed-back loops can be formed in neuronal networks and what roles they can play. In vertebrates, the piriform cortex, part of the olfactory cortical complex, sits in a position equivalent to the mushroom bodies. “It is very likely that mammals have similar all-to-all control mechanisms in cortical and other circuits. They might not consist of single interneurons, however, but rather of populations of inhibitory neurons with means to couple their responses and actions”, surmises Laurent. “Insect brains never cease to give us insights about neural computation, and to put elegant solutions right on our laps, if we know where to look and are a bit lucky.”
(HR)