Electrons discover their individuality
Scientists in Berlin observe the change from cooperative to individual behaviour in electrons with different speeds
Electrons have something in common with people: the more information they acquire about their setting, the more they become aware of their individuality and the more belonging to a group loses its importance. As a result, the coherent harmony that binds the electrons into a fixed relationship with their environment is lost. This is what scientists at the Fritz-Haber Institute of the Max-Planck Society discovered when, with the aid of X-rays, they catapulted electrons out of molecules consisting of two nitrogen atoms. If in the process an electron is only slightly accelerated, it does not recognize from which of the two atoms it has been ejected and therefore behaves as if it had come from both atoms: It acts like a pseudo-pair that is fully cooperative. On the other hand, if the electron is accelerated quickly enough it knows whence it came and exhibits the characteristics of an individual. As the behaviour changes from cooperative to individual, the transition from quantum physics to classic physics can be explored. Furthermore, such transitions also play a role in technically interesting materials such as superconductors and magnets, and artificial molecules, - consisting of quantum points, which are intended to process data as components of future quantum computers. (Nature Physics, Vol.4 No.8 August 2008)
In superconductors, electrons give up their solitary behaviour and combine into pairs. These "Cooper pairs" conduct current without resistance. In magnetic materials in fact, all of the electrons, every one of which resembles a small bar magnet, are aligned as if in military formation. In order to understand such materials more thoroughly and possibly to improve their properties, physicists want to find out how electrons switch from cooperative to individual behaviour and vice-versa.
Uwe Becker and his colleagues at the Berlin Fritz-Haber Institute of the Max-Planck Society have now studied this behaviour in individual electrons by ionizing a nitrogen molecule with X-rays of increasing energy. As the energy of X-ray radiation increases, the kinetic energy and speed of the ejected electrons increase. In quantum mechanics, where the electrons appear simultaneously as particles and as waves, this means that their wavelength, which physicists call the de Broglie wavelength, becomes shorter: the higher the energy of the wave, the shorter its wavelength.

In the same way that the wavelength of electromagnetic waves decides which details can be perceived in their vicinity, the perceptive capacity of the electrons improves as their de Broglie wavelength decreases. The greater the energy, the more information they acquire about their surroundings.
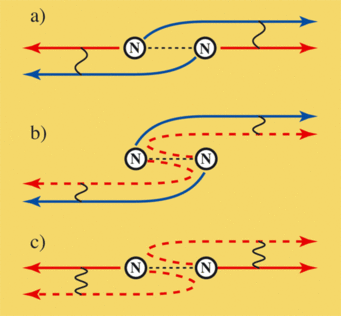
In the experiment conducted by the Berlin-based physicists this was made very clear when the electron’s wavelength decreased to below the distance between the two atoms of the nitrogen molecule. As these two atoms are identical, the X-ray beam does not distinguish between out of which atom it catapults the electron. As long as the wavelength of the departing electron is greater than the distance between the atoms, the electron does not distinguish from which atom it came. It does not even know its place of origin.
And what’s more, an electron even exhibits the features of a pair of electrons whose partners have started from both atoms. In reality the emitted electron constantly tunnels between the two atoms - a quantum mechanical behaviour that opens paths to particles which, in classical physics, are blocked to them for energetic reasons. During tunnelling, the electron jumps extremely quickly between the two nitrogen atoms. "If it then leaves the molecule with low kinetic energy (relatively slowly), it cannot tell from which atom it actually departed," explains Uwe Becker. In other words, the individual atom acts like a pseudo pair, the halves of which each start from one atom - an amazing phenomena that is only possible in quantum physics.
The fact that the electron actually begins its journey as an electron pseudo pair is recognized by physicists through a characteristic intensity pattern generated by the two halves of the electron when detected. As they behave like waves, they superimpose themselves to form a characteristic interference pattern - just like the waves from two stones falling into a pond superimpose themselves on each other. Such an interference pattern not only proves that an electron started from two atoms but also demonstrates the phase-coupled and the cooperative behaviour of the two "halves" of the electron.
"This special interference pattern is no longer observed if the speed of the emitted electron exceeds a particular value," says Uwe Becker. The de Broglie wavelength then decreases to below the distance between the nitrogen atoms and the electron can now detect from which atom it was emitted, like a "Heisenberg microscope." Werner Heisenberg came up with the idea of such a microscope which he assumed able to localize particles through an energy-dependent impact process via a blurred relationship and thereby resolve them locally. As such a microscope could not be developed during his lifetime, he later distanced himself from his proposal. "Our experiment is a rare example of such a microscope" explains Uwe Becker.
As the higher-speed electrons now know their origin, another effect comes into play. Shortly after the electron is emitted or spreads out in wave form it hits the second atom - but can now distinguish between the two atoms. Such an obstacle in the path of a wave acts as a starting point for a new wave front. Physicists call this scattering. This means that an electron wave now travels in the same direction - in its originally unscattered and in the scattered version.
The two waves of the original and scattered electron of the same atom superimpose themselves in place of the two waves emitted from different atoms. Physicists use this effect in order to explain the structure of solid substances in a process called "Extended X-ray Fine Structure" (EXAFS). Here an interference pattern also occurs that provides information about the nature and arrangement of the scattering atoms. However, this pattern differs from that of the two "halves" of an electron emitted from the two atoms with low energy, as the scattered electron no longer cooperates with its adjacent electron but only with an unscattered predecessor. Uwe Becker describes this as follows: "The waves from both sides are no longer coherent and therefore no longer have a fixed phase relationship with each other." The latter simply means that the peaks and troughs of the two waves are no longer at a fixed distance from each other. Coherence is lost as the electron now knows from which atom it originates: it behaves egocentrically.
Uwe Becker draws a parallel between this physical phenomenon and certain social developments to more clearly explain the transition from cooperative to egocentric behaviour of electrons: "As soon as people acquire more information about themselves and their environment, they start to reflect on their situation" explains the physicist. Their behaviour, which was previously fully in coherent harmony with their surroundings, that is the collective group, becomes more individual. This transition can currently be observed in various cultures, particularly in the Middle East. The reason why the Taliban, for example, tried to withhold education and information in such simple forms as popular music and movies, is the fear of this inevitable process, which fundamentally threatens the existence of the traditional coherence-based social structures. However, this fear-tinged attitude fails to recognize the opportunities that a knowledge-based society offers for the future of an, albeit egocentric, yet united society, according to Uwe Becker.