Perilous Puddles
Admittedly, the research subject isn’t particularly appetizing: Strongyloides stercoralis – small parasitic worms that live in their host’s intestines and have the potential to cause severe problems. Nevertheless, Adrian Streit from the Max Planck Institute for Developmental Biology in Tübingen is fascinated by this threadworm. It has a unique life cycle, and to this day, no one really understands why

Text: Catarina Pietschmann
Referring to nematodes as unusual is almost an understatement, as strange behavior is completely normal for them. Pristionchus pacificus, which lives, among other places, on the Pacific island of La Réunion, seeks out a beetle larva, climbs up onto it and then stops developing. As soon as the beetle dies, the worm continues its development, gorges itself on the carcass and multiplies (MaxPlanckResearch 2/2014).
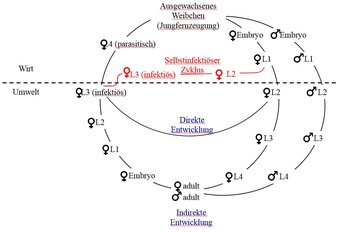
But compared with Strongyloides, that’s almost boringly conventional. At the Max Planck Institute for Developmental Biology, Adrian Streit explores how this worm can survive in two worlds. Between parasitic generations, Strongyloides can also form free-living generations. Parasites are exclusively females that multiply in the host’s intestines by parthenogenesis. “They produce both male and female eggs, which are excreted with the feces,” explains Streit. “Either infectious larvae develop from the female eggs, which then immediately crawl back into the host, or free-living worms that mate with males are produced.”
Parasitic females

The male worms are exclusively free-living. If males and females reproduce in the soil, only parasitic female offspring are formed. This second generation of larvae thus needs to find another host to be able to multiply – which then once again takes place without a male partner.
Many terrestrial vertebrate species have their own Strongyloides – including humans. The World Health Organization estimates that more than 300 million people worldwide are infected with the threadworm, especially in northern South America, Central Africa and Asia. A warm, humid climate and a lack of hygiene are an El Dorado for worms!
In healthy people, the infection usually goes undetected, as they harbor only comparatively few worms. In immunocompromised patients, however, the larvae in the intestines can infect other organs and cause a life-threatening infection called strongyloidiasis. “If the parasite isn’t detected in cancer patients, for example, chemotherapy can end in disaster,” emphasizes Streit.
Organ recipients are also at risk. Even in Western countries, deaths have occurred after transplants as a result of the worms. In the Netherlands, two cases were reported in which the infection was proven to have been transmitted via donated organs – the donor had lived in South America 20 years previously. “In this country, the worms are not yet a major medical problem, but these cases are reason enough to take a closer look,” says Streit.
It isn’t unusual for the infection to remain undetected for a long time, as it can be completely symptom-free. In addition, the classic symptoms – skin rash, nausea, diarrhea, abdominal cramps – are unspecific, so it’s easy to overlook the worms. The tragedy is that common worm remedies would have been sufficient to kill the parasites.
A lack of sanitary hygiene results in the worm being transmitted from person to person. But is this really the only way? Adrian Streit is driven by the question of whether strongyloidiasis is one of the zoonoses, meaning that it can be transmitted from animals – dogs, for example – to humans. If this is the case, worms with identical DNA would have to be found in both dogs and their owners.
Field study in Cambodia

To learn more about the transmission paths, Streit has teamed up with the Swiss Tropical and Public Health Institute in Basel and the Cambodian National Center for Parasitology, Entomology and Malaria Control. The two institutes maintain a field laboratory in northern Cambodia. The rural region is ideal for this purpose: the farmers’ houses are built on stilts – the family lives upstairs, and the animals, generally pigs and dogs, live below. The sanitary facilities are anything but hygienic and residents go barefoot or wear open sandals.
Nematodes survive for weeks in the moist, feces-contaminated soil. The larvae bore into the skin and advance at ten centimeters per hour – pretty fast for creatures less than one millimeter long! That’s why medical experts respectfully refer to them as “racing larvae.” Skin irritations usually occur in the vicinity of the worms.
They then bore through the wall of a blood vessel and are flushed into the lungs with the blood. Here, they penetrate the tissue and migrate upward through the trachea. “First coughed up, then swallowed – that’s how they enter the digestive tract,” explains Streit.
In the small intestine’s mucous membrane, each female lays up to a thousand unfertilized eggs per day. The majority are excreted in the feces. However, this human parasite has the unpleasant characteristic that a percentage of the embryos develop into infective larvae within the host. These penetrate the intestinal wall or bore through the anal mucous membrane and reenter the body. This is why, if left untreated, the infection can persist for a very long time.

In Cambodia, with the consent of the villagers, Streit’s doctoral students Tegegn Jaleta and Siyu Zhou took fecal samples from humans and animals. “This was a huge event for the people there,” says Streit, smiling. “Many came to help.” People were treated free of charge by staff from both institutes, and it was explained how they could protect themselves from infection.
The samples collected were first incubated for two days and placed in water, after which the worm larvae swimming in the water were separated out. However, genetic analyses weren’t possible in the poorly equipped field laboratory in the village. The scientists thus had to fly the worms out to Germany, placing each one into a small tube containing ethanol. “Customs wasn’t overly enthusiastic, but the officials were reassured once it became clear that the worms weren’t being shipped alive and were in sterile packaging,” explains Streit.
Streit and his colleagues then examined the worms’ genetic material in their laboratory in Tübingen. The analysis revealed that one of the two Strongyloides populations that the researchers had identified in the dog feces was genetically identical to that in their owners’ excrement. In other words, the populations overlap, so dogs must be seriously considered as carriers.
As a next step, Streit wants to investigate whether dogs are the only carriers for humans. Water buffalo may also be candidates, as peasants in many areas still plough their rice fields with the animals, and do so barefoot. Water buffalo are already known to be carriers of other zoonotic diseases; studies in southern China, for example, revealed that the animals were the main carriers of schistosomiasis, a disease caused by trematodes.
“Although treatment of the patients was quite successful, the worms could hardly be suppressed by treating humans alone. Nevertheless, the infection was brought under control in China, but only once the water buffalo were also dewormed,” says Streit.
Could this be a model for dealing with strongyloidiasis? To find this out, Streit is planning a project similar to the one in Cambodia, but this time in southwestern China. Here, not only are there rural areas where the threadworms are abundant, but there are also highly qualified scientists with well-equipped laboratories.
Relevance to veterinary medicine

A worm infection can be identified under the microscope. With more than 25,000 species worldwide, nematodes are hardly distinguishable for the layperson, but not for biologists. “Apart from Parastrongyloides, a close relative, Strongyloides is the only nematode in which the infective larvae have a very long esophagus typical of these species.
However, the fecal samples contain predominantly other nematodes, such as hookworms, as both humans and animals in Asia are often infected with various worm parasites. It remains unclear whether the different parasite species compete in the intestines. “But one thing is certain: in a further infection, worms can suppress new arrivals of the same species,” explains Streit. “We don’t yet know how, but this could offer an approach for future treatment.”
To discover the worms’ tricks, Streit keeps two other Strongyloides species in Tübingen, where they live in rats or sheep (or, in the lab, in rabbits). Together with the University of Hohenheim, which maintains an animal breeding station in the Swabian Alb, he can also analyze the natural sheep parasite population for comparison.
In contrast to parasitic nematodes, Strongyloides doesn’t play a major role in veterinary medicine. Unlike Strongyloides stercoralis, other Strongyloides species don’t lead to long-lasting, self-sustaining infections. However, these species, which can also live outside the host, are suitable as study objects for basic biological research.

Streit also wants to investigate whether the threadworms possess something like “parasite genes” – that is, a group of genes that are necessary for this lifestyle. In 2016, scientists decrypted the genomes of four different Strongyloides and two other closely related species, one of which occasionally lives parasitically, while the other is free-living. A genome comparison revealed that the parasitic worms have more genes for two protein families that suppress the host’s immune response than their free-living relatives do. “We don’t yet know what role these genes play in a parasitic lifestyle,” Streit emphasizes. “For this, we would need to switch off the individual genes – not very easy in an organism that lives in a host every second generation.”
An additional peculiarity of these worms is that the free-living, bisexual generation produces exclusively female offspring, while the unisexual parasitic generation produces both males and females through parthenogenesis. Depending on the Strongyloides species, one chromosome must be completely or partially degraded so that males can develop without a father. The controlled degradation of genetic information is called chromatin diminution and was first identified in the equine roundworm. Such degradation is a rare occurrence in nature – outside of nematodes, it occurs, for example, in copepods, ciliates and lampreys.
Generation without males

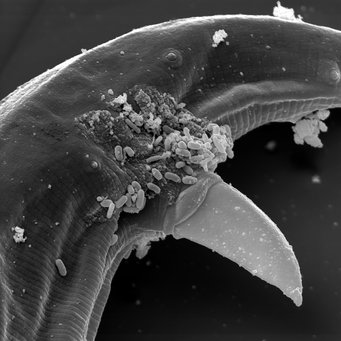
The copulation organ of a free-living male Strongyloides papillosus. The small rod-shaped structures are bacteria clinging to it.
Different Strongyloides species also appear to have different means of preventing males from developing in the progeny of the bisexual, free-living generation. Similar to many nematodes, the females of the rat parasite also have two X chromosomes, whereas the males have just one. A Y chromosome as in humans doesn’t exist in these species.
“While we didn’t find mature sperm leading to males in sheep worms, there are sperm with and without an X chromosome in rat parasites,” Streit explains. Some of these sperm would therefore have to lead to males, since an egg cell always carries an X chromosome. In contrast to the sheep, there are actually male worm embryos in the rat. However, they apparently die off, because neither of the two worm species forms male larvae. Still, when the male-designated sperm or the early male embryos are screened out remains a mystery.
Why is a parasite’s life so complicated? As Adrian Streit sees it, the complex life cycle emerged gradually from a simpler one: numerous free-living nematodes occasionally form permanent stages to survive hard times. They often attach themselves to other animals, such as the previously mentioned worm living on a beetle. “Once such a larva is attached to an animal, the step to get inside the animal is no longer that great. This may lead to a life cycle similar to that of Strongyloides, where the worm is either parasitic or free-living,” explains Streit. However, in the majority of parasitic nematodes, every generation is parasitic. Thus, in the course of evolution, the free-living phase may have been sacrificed in favor of a purely parasitic one in many parasites.
Strongyloides may be following the same path. But has it possibly taken a wrong turn? After all, the females multiply in their hosts exclusively by unisexual parthenogenesis, so “rejuvenation” of the genome by gene recombination doesn’t take place in the parasitic generation.
In evolutionary terms, unisexually or asexually reproducing lines are generally young. It may even be assumed that they can’t age, because the transition to a life without sexual reproduction is the beginning of the end. In other words, Strongyloides may have maneuvered itself into a cul-de-sac, making it impossible for it to now rescind the sexual free-living cycle.
But maybe the worm has found an ideal solution for itself: thanks to the two life cycles, a single, self-reproducing parasitic individual can establish a new population without sacrificing the benefits of sexual reproduction.
Whether and how Strongyloides will ever become a pure parasite, or whether its life cycle will become even more complicated, can’t be predicted with certainty today. Evolution always finds a new, and sometimes curious, solution.
To the Point
-
There are more than 50 species of parasitic Strongyloides (a threadworm) that infect a wide range of terrestrial vertebrates. An estimated 300 million people worldwide are infected with the Strongyloides stercoralis worm
-
Dogs can also be infected with Strongyloides stercoralis. The parasites can infect people through the animals’ feces. The infection is usually harmless in humans, but it can end in death in individuals with a weakened immune system.
-
Genetic analyses have revealed that parasitic worms have more genes compared with free-living species, potentially reducing the host’s immune response.