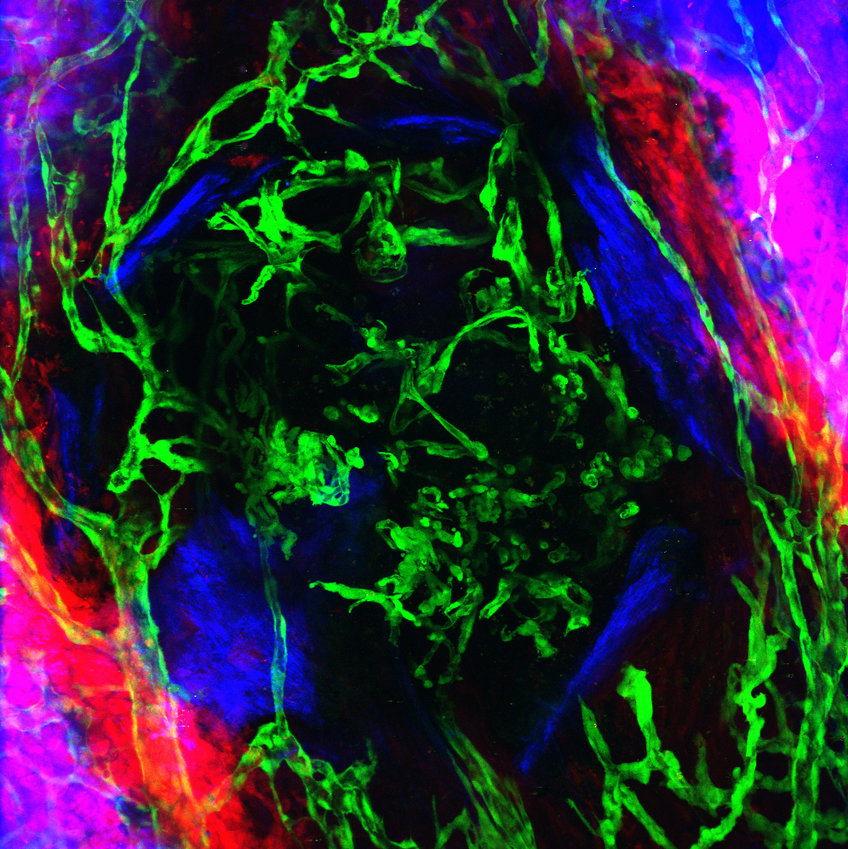
Blood vessels are the pioneers of bone formation in the skull
New long-term microscopy method shows differences to long bones
Living bone is fascinating because of its unique ability to adapt to mechanical stress and regenerate without scarring. During fracture healing, blood vessels and bone cells work closely together to gradually replace the initial cartilaginous wound tissue with ingrowing blood vessels and new bone tissue. The bone progenitor cells closely follow the course of the newly formed vessels, an observation known as angiogenic-osteogenic coupling. This is how a broken leg heals. But what happens when the skull is injured? A team of researchers at the Max Planck Institute for Molecular Biomedicine in Münster, Germany, has succeeded for the first time in using a highly specialized laser microscope to observe the healing of skull bone and the growth of new blood vessels without co-migrating bone precursor cells. The vessels initially grow alone without bone precursor cells in order to prepare the injured bone tissue for subsequent ossification. This type of bone healing is therefore fundamentally different from previously known processes in tubular bones.

Blood vessels are essential for delivering vital oxygen and nutrients to the body. In the skeletal system, blood vessels have a specialized morphology and penetrate the bone as a dense vascular network. They regulate the formation of bone precursor cells through the release of signaling molecules, and thus the formation, maintenance, and regeneration of bone tissue.
The healing of tubular bones, or long bones in the arms and legs, is an area of intense research in orthopedics and trauma surgery. Flat bones, such as those found in the skull, differ from long bones in that they do not carry weight. Further differences are found during growth and development of these types of bone. Scientists at the Max Planck Institute have now investigated whether the findings on bone healing in long bones can be transferred to flat bones or whether there are differences.
To observe the growth of new blood vessels during the healing of skull bone, a team of Max Planck researchers has developed an intravital microscopy method that allows them to follow the sprouting of vasculature and the ingrowth of new bone in vivo for over a month. Using a multiphoton microscope, which is specialized on intravital studies, the scientists were able to penetrate deep into the regenerating tissue and visualize vascular and bone cells as well as fibers of bone matrix collagen with high resolution.
Gabriele Bixel, first author and project leader of the study together with Ralf Adams, explains: "With our new experimental approach, we were able to take intravital images at one and the same site over several weeks and thus follow the healing of the cranial bone injury from the beginning to the end of the healing process."
The scientists discovered: "During the healing of a cranial bone lesion, the sprouting vessels did not grow in close proximity to bone precursor cells as we know it from long bones. To our surprise, the regenerating vessels first grew into the bone wound alone, like pioneers, and established a primitive blood supply. Only when the supply of oxygen and nutrients is guaranteed will the bone cells migrate to the injured bone site as a multi-cellular sheath and will gradually begin to ossify the lesion," explains Bixel.
cartilage cuff at the fracture site
This type of healing of the skull bone is fundamentally different from the healing of a femur fracture. "A broken femur heals by first forming a soft callus, a cartilage cuff, around the fracture site. This callus of cartilage cells forms a temporary stabilizing structure around the broken bone," explains Bixel. As the bone heals, this soft callus is gradually transformed into bone tissue on both sides, starting from the outer ends, by ingrowing vessels with co-migrating bone progenitor cells. The bone progenitor cells follow the course of the newly formed vessels in immediate vicinity," says Bixel.
The current study examined small injuries to the skull bone. "We cannot yet conclude what role regenerating blood vessels play in the healing of large bone defects or deep skull fractures, such as a skull base fracture," says Bixel. "Another exciting question for us is how vascular and bone cells communicates with each other and grow together into the injured bone, and how and why this angiogenic-osteogenic coupling is abolished during the healing of small injuries to the skull bone," says Bixel.
Understanding the vasculature and its central role in bone healing is critical to developing effective strategies to improve bone regeneration. This is still one of the major challenges that orthopedic surgery has to face today.
The interdisciplinary study was carried out in collaboration with Melanie Timmen and Richard Stange from the Department of Regenerative Musculoskeletal Medicine at the Institute for Musculoskeletal Medicine of the University of Münster.