Visual proteomics – on the way to a three-dimensional protein atlas
If proteins are to fulfil their functions in a cell, they need to be in the right place at the right time. In addition, they often join forces and form complexes made out of several thousand proteins. Wolfgang Baumeister, Director at the Max Planck Institute of Biochemistry in Martinsried, and his team want to find out how proteins are distributed in a cell and what components make up protein machines. To do this, they use the cryo-electron tomography technique developed by them to take three-dimensional images of cells and their proteins.

Several thousand different proteins are at work in any one cell. To ensure that they interact smoothly, each of these molecules needs to cooperate with the right partners. With the aid of cryo-electron tomography (cryo-ET), Wolfgang Baumeister and his colleagues at the Max Planck Institute of Biochemistry were able to visualize the proteins of a cell and investigate their spatial arrangement.
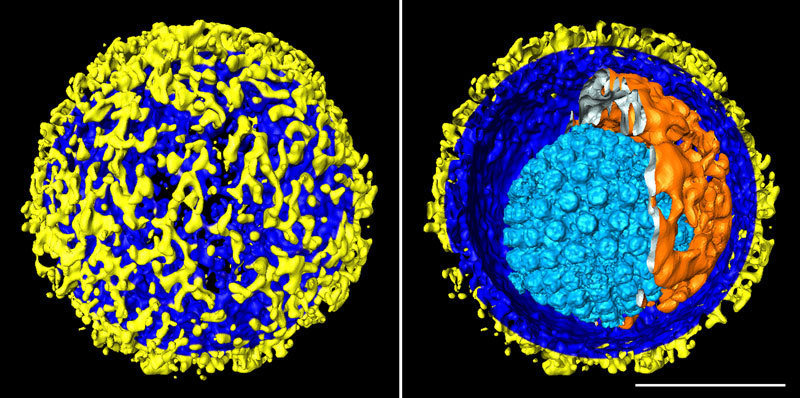
The Max Planck researchers have succeeded in taking a series of fascinating snapshots with cryo-ET. Thus, for example, they generated 3D images of the herpes simplex virus, with a three-dimensional visualization of the structure of the whole virus including the layers that make up its shell. The images show how a protein shell with icosahedral symmetry – known as the capsid – encloses the virus’s genetic material. The capsid, in turn, is surrounded by the tegument which, finally, is enclosed by the envelope. Hundreds of protein projections of varying shape and size protrude from the envelope, enabling the virus to infect the body’s cells.
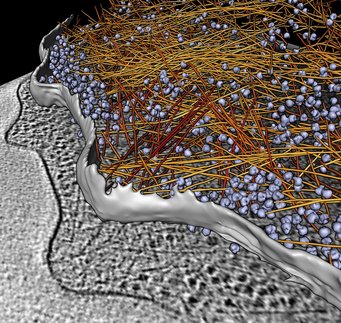
3D reconstructions of the synapses of nerve cells show how the tiny blisters, known as vesicles, which contain the messenger substance that transmits signals, are held in place by thread-like proteins. These protein threads bind the vesicles to each other and keep them in the active zone of the synapse until they receive the signal to fuse with the cell membrane and release their contents.

In addition, the scientists have investigated the nuclear pores, i.e. the protein complexes through which molecules are transported into or out of a cell’s nucleus. The resolution of these images is so high, that even the molecules being channelled into the nucleus are visible.
The cell’s protein factories, the ribosomes, have also revealed their three-dimensional structure and their mode of operation thanks to cryo-ET. Thus, the researchers have discovered in what patterns the ribosomes come together to allow them to read out the messenger RNA, i.e. the assembly instructions, and to produce proteins. To do this, they align themselves in such a way as to ensure that the synthesized proteins have enough space to fold correctly. Moreover, the scientists have used cryo-ET to document the special arrangement of bacterial ribosomes when they are not in operation. These images show that the ribosomes arrange themselves side by side in pairs, as soon as the bacteria are unable to find food. In this stand-by position, they bide their time until sufficient food is available to resume protein production.
Having photographed the already minute viruses, synaptic vesicles and other cell organelles, the scientists want to do the same with even smaller objects, namely individual protein molecules. These may hold valuable information for future use in medicine, for example. In this context, Felix Bäuerlein, a doctoral student in Wolfgang Baumeister’s Research Group, wants to use the method to study the nerve cells of Alzheimer’s disease patients. In this form of dementia, some proteins clump together and form deposits in the cells. “If we know the structure of these protein plaques, we may also find out why they cause so much damage to the cells”, hopes the researcher.

To ensure that the protein images are as lifelike as possible, the first thing the researchers have to do is to “capture” the samples in their original state. They do not use chemicals to do this, as these would alter the structure of the proteins. Instead, they rely on flash-freezing: they use a guillotine-like device to shoot the sample at lightning speed into an ethane bath, surrounded by liquid nitrogen at minus 196 degrees. When dipped into this bath, the sample freezes within a few thousandths of a second. This abrupt chilling does not give the water time to crystallize. Instead, a layer of what is known as amorphous ice is formed around the sample. In this type of ice, the water molecules are arranged randomly, rather than regularly as they would be in a crystal. This is crucial, as ice crystals would alter or even destroy the structures in the cell.
The electron microscope which the scientists then use to analyse the samples is among the most cutting-edge ones in the world. Measuring several metres in height, this monster reaches almost up to the ceiling of a foam-lined room in the Institute’s cellar. The purpose of the foam is to cushion any vibrations or shocks while the microscope is in operation, since even footsteps in the corridor or a door banging would distort the micrographs. A total of four such microscopes are located at the Max Planck Institute in Martinsried.
To stop the flash-frozen specimens from thawing out, they are constantly cooled with liquid nitrogen, even while in the microscope. The reason is that the samples must not warm up to more than minus 136 degrees, since destructive ice crystals would otherwise be formed. In the microscope, the sample is positioned on a tilting mount. This allows the scientists to tilt the object through 140 degrees and take about 150 pictures in the process. “After each picture is taken, the microscope recalibrates itself automatically and is refocused. By doing so, it compensates for tiny movements of the specimen between two shots”, explains Wolfgang Baumeister. Together with colleagues, he developed this automatic image generation method in the late 1980s.

It is only thanks to this automation that scientists can produce such extensive image series, while keeping the radiation dose so low that even biological objects are not damaged. It takes one to two hours for one series to be completed. The resultant flood of data can only be managed with the aid of sophisticated software. Complicated computer algorithms search the photographs for reference points, which they use to align the individual images. The computer then uses the combined individual micrographs to generate a three-dimensional image.
Cryo-ET is simplest to use on particularly thin objects, such as bacterial cells. This is because the electron beam achieves good penetration only in specimens with a thickness of around 500 millionths of a millimetre – corresponding to about one hundredth of a human hair. Mammalian cells, however, are considerably thicker. To enable them to examine proteins in these cells as well, the Max Planck researchers are using a technique from materials science: in a so-called focused ion beam microscope, they bombard the surface of the specimen with a high-energy beam made of gallium ions. This can be imagined as being similar to a meteorite shower: on impact, the heavy gallium particles knock out the uppermost atoms from the surface of the sample. Individual layers are thus removed without crushing the cell, as can happen with conventional cutting methods.
Wolfgang Baumeister and his team are continuing to develop and refine their technique. The resolution of the three-dimensional images has now become so fine, that two points only three millionths of a millimetre apart can be distinguished on them. At present, one difficulty in the analysis is that it is tricky to make interesting structures stand out against the background: because the electron dose has to be very low for biological specimens, the images are underexposed, as it were. However, increasingly more sensitive detectors that can manage with fewer electrons are now being developed. “With these, we can take an increased number of individual images using the same radiation dose”, says Wolfgang Baumeister. The precision of the visualization increases with the number of superimposed images.